MFA-4-NO2: 2-((2,3-dimethyl-4-nitrophenyl)amino)benzoic acid (C15H14N2O4)
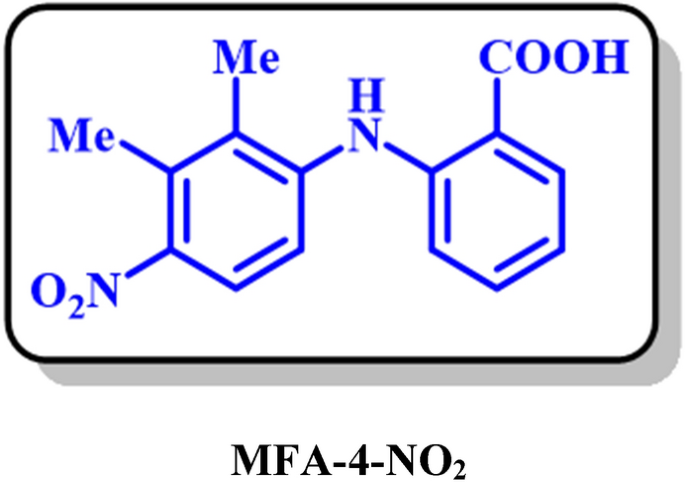
Isolated yield: 57%. M.p.: 278–281. 1H NMR (500 MHz, DMSO-d6) δ (ppm): 2.27 (s, 3H, aliphatic), 2.44 (s, 3H, aliphatic), 6.81 (t, J = 8.0 Hz, 1H, aromatic), 7.13 (d, J = 7.1 Hz, 1H, aromatic), 7.24 (t, J = 7.5 Hz, 1H, aromatic), 7.35 (d, J = 8.8 Hz, 1H, aromatic), 7.74 (d, J = 8.8 Hz, 1H, aromatic), 7.94 (d, J = 7.8 Hz, 1H, aromatic). 13C NMR (125 MHz, DMSO-d6) δ (ppm): 14.1 (C-15), 16.5 (C-13), 110.9 (C-6), 115.7 (C-9), 119.2 (C-4), 123.3 (C-10), 129.3 (C-3), 131.7 (C-5), 126.1 (C-12), 146.5 (C-7), 142.9 (C-11), 111.2 (C-2), 116.4 (C-14), 125.9 (C-8), 169.6 (C-1). IR (KBr) ν (cm−1): 3243 (N–H, O–H), 3075, (weak, C–H, aromatic), 2924, 2855 (weak, C–H, aliphatic), 1670 (weak C=O), 1497 (strong, C=C), 1578, 1383 (strong, N=O), 1297 (strong, C–O), 1171, 751. MS (EI, 70 eV): m/z (relative intensity) 286 (M+·, 45), 222 (40), 167 (64), 149 (100), 76 (50).
MFA-5-NO2: 2-((2,3-dimethyl-5-nitrophenyl)amino)benzoic acid (C15H14N2O4)
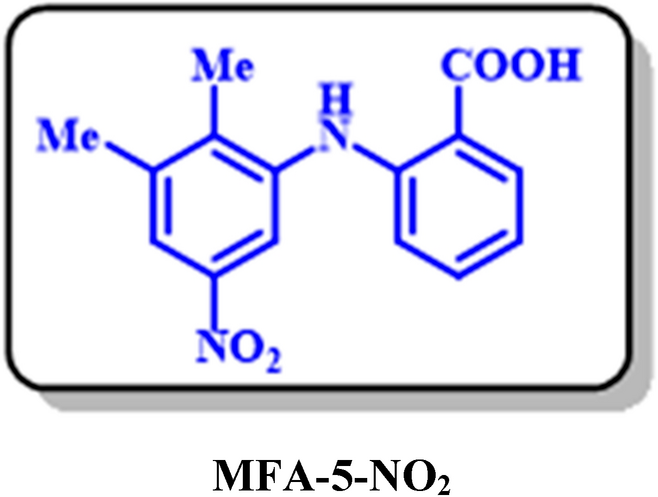
Isolated yield: 32%. M.p.: 278–281. 1H NMR (500 MHz, DMSO-d6): δ 2.28 (s, 3H, aliphatic), 2.34 (s, 3H, aliphatic), 6.65 (t, J = 8.65 Hz, 1H, aromatic), 7.68 (d, J = 8.0 Hz, H, aromatic), 6.58 (t, J = 8.0 Hz, H, aromatic), 76.98 (t, J = 7.9 Hz, 1H, aromatic), 7.86 (d, J = 7.9 Hz, 1H, aromatic), 7.02 (t, J = 7.0 Hz, 1H, aromatic). 13C NMR (125 MHz, DMSO-d6): δ 15.08 (C-15), 20.46 (C-13), 110.9 (C-6), 119.24 (C-4), 131.72 (C-5), 126.14 (C-11), 129.29 (C-3), 132.92 (C-8), 129.6 (C-9), 146.49 (C-7), 141.77 (C-12), 111.16 (C-2), 126.35 (C-14), 125.99 (C-8), 169.65 (C-1). IR (KBr) ν (cm−1): 3243 (broad, N–H, O–H), 3075, (weak, C–H, aromatic), 2924, 2855 (weak, C–H, aliphatic), 1670 (weak C=O), 1497 (strong, C=C), 1578, 1383 (strong, N=O), 1297 (strong, C–O), 1171, 751. MS (EI, 70 eV): m/z (relative intensity) 286 (M+·, 45), 222 (40), 167 (64), 149 (100), 76 (50).
1S-RED: 2-((4-((2-hydroxynaphthalen-1-yl)diazenyl)-2,3-dimethylphenyl) amino)benzoic acid (C25H20N3O3)
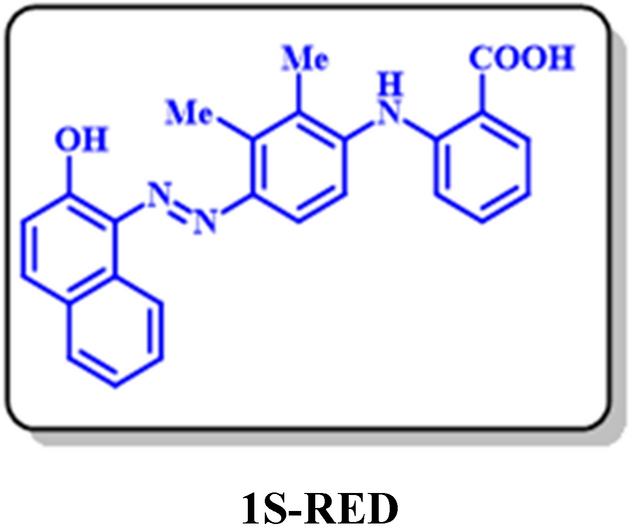
Isolated yield: 46%. Mp > 300 °C (Dec.). 1H NMR (500 MHz, CD3OD) δ (ppm): 2.35 (s, 3H, aliphatic), 2.53 (s, 3H, aliphatic), 6.79 (t, J = 8.0 Hz, 1H, aromatic), 7.01 (d, J = 8.9 Hz, 1H, aromatic), 7.19 (d, J = 8.9 Hz, H, aromatic), 7.56 (t, J = 7.6 Hz, 1H, aromatic), 7.72 (d, J = 8.3 Hz, 1H, aromatic), 7.82 (d, J = 9.0 Hz, 1H, aromatic), 7.92 (d, J = 9.2 Hz, 1H, aromatic), 7.98 (d, J = 7.8 Hz, 1H, aromatic), 8.7 (d, J = 8.5 Hz, 1H, aromatic), 8.55 (s, 1H, aliphatic). 13C NMR (125 MHz, CD3OD) δ (ppm): 18.82 (C-15), 29.43 (C-13), 113.98 (C-6), 115.02 (C-9), 116.75 (C-4), 117.73 (C-10), 121.19 (C-24), 121.7 (C-20), 124.58 (C-19), 127.9 (C-21), 128.15 (C-18), 130.5 (C-3), 131.66 (C-5), 136.61 (C-23), 122.5 (C-2), 127.76 (C-14), 128.11 (C-17), 129.63 (C-16), 132.08 (C-22), 133.13 (C-13), 139.84 (C-16), 142.67 (C-11), 144.71 (C-7), 174 (C-1). IR (KBr) ν (cm−1): 3301 (broad, N–H, O–H), 2923, 2852 (medium, C–H, aliphatic), 1384 (strong, N=N), 1727 (weak C=O), 1583 (strong, C=C), 1277 (strong, C–N),1075, 824. MS (EI, 70 eV): m/z (relative intensity) 411 (M+·, 3.8), 341 (7.69), 149 (66), 69 (100), 41 (55.5).
2S-RED: 2-((5-((2-hydroxynaphthalen-1-yl)diazenyl)-2,3-dimethylphenyl)amino)benzoic acid (C25H20N3O3)
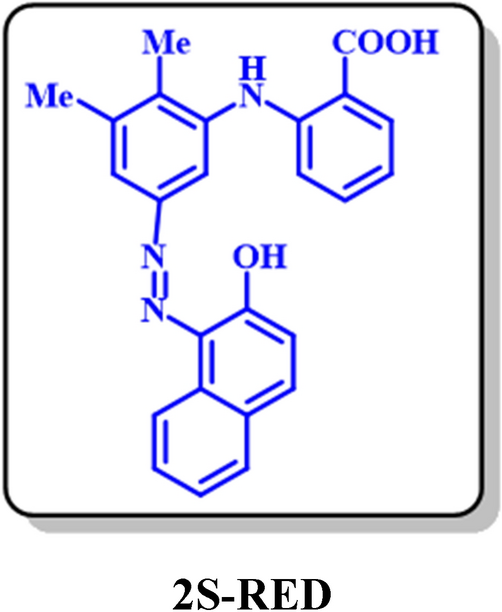
Isolated yield: 41%. Mp > 300 °C (Dec.). 1H NMR (500 MHz, CD3OD) δ (ppm): 2.23 (s, 3H, aliphatic), 2.34 (s, 3H, aliphatic), 6.89 (d, J = 8.9 Hz, 1H, aromatic), 7.03 (d, J = 7.3 Hz, 1H, aromatic), 7.12 (t, J = 8.0 Hz, 2H, aromatic), 7.21 (d, J = 8.1 Hz, 1H, aromatic), 7.4 (t, J = 8.5 Hz, 1H, aromatic), 7.58 (t, J = 8.0 Hz, 1H, aromatic), 7.78 (d, J = 8.0 Hz, 1H, aromatic), 7.81 (t, J = 8.0 3H, aromatic), 8.64 (d, J = 5.5 Hz, 1H, aromatic), 8.56 (s, 1H, aliphatic), 8.86 (d, J = 8.7 Hz, 1H, aromatic). 13C NMR (125 MHz, CD3OD): δ 12.83 (C-15), 19.22 (C-13), 113.1 (C-17), 119.9 (C-6), 121.51 (C-11), 121.9 (C-4), 123.9 (C-19), 125.52 (C-10), 125.99 (C-23), 127.08 (C-21), 127.66 (C-22), 128.4 (C-24), 133.41 (C-3), 137.81 (C-5), 150.31 (C-16) 139.91 (C-7), 138.73 (C-13), 132.83 (C-8), 131.29 (C-20), 126.92 (C-9), 125.6 (C-24), 123.8 (C-25), 123.6 (C-2), 169.1 (C-1). IR (KBr) ν (cm-1): 3301 (broad, N–H, O–H), 2923, 2852 (medium, C–H, aliphatic), 1727 (weak C=O), 1583 (strong, C=C), 1383 (strong, N=N), 1277 (strong, C-N), 1075, 824. MS (EI, 70 eV): m/z (relative intensity) 413 (M+·, 5.8), 368 (12), 239 (18), 149 (41), 43 (100).

